Coronavirus Files: POC miss out on business loans; vaccine plans for the less-than-eager

Since last April, The Center for Health Journalism has been publishing a special newsletter geared to journalists as they report on one of the biggest and most complex stories of our times. Each Monday, while the pandemic runs its course, The Coronavirus Files will provide tips and resources and highlight exemplary work to help you with your coverage. This week, the Center for Health Journalism’s Coronavirus Files Monday newsletter is curated and reported by science writer Amber Dance, PhD. Have a suggestion or a request? Write us at editor@centerforhealthjournalism.org.
Business owners of color less likely to benefit from federal aid
Black-, Latinx-, and Asian-owned businesses were much less likely than white-owned ones to benefit from the federal Paycheck Protection Program, according to an in-depth investigation by the Los Angeles Times and Reveal (a platform of the Center for Investigative Reporting). PPP loans were supposed to help small businesses and independent entrepreneurs as well as large corporations, and Congress ordered priority for underserved areas. According to the investigators’ analysis of more than 5 million loans, that didn’t happen. In large metro areas across the nation, white-owned businesses were more likely to score funds. In Los Angeles, for example, five major banks made loans to almost one-quarter of businesses in majority-white regions, 20% in Asian areas, 16% in Black communities and 11 % in Latinx regions. Banks, overwhelmed by applications, “failed to live up to the goals of a 44-year-old federal law that requires them to equitably serve all communities where they do business,” according to Jesse Van Tol, CEO of the National Community Reinvestment Coalition, a fair-lending group. And Kevin Stein of the California Reinvestment Coalition noted that because business ownership is a key method to build wealth, the effects are likely to ripple through communities of color for generations.
Vaccination programs pivot with July 4 hopes
If people keep getting vaccinated and viral variants don’t throw a kink in the works, a new CDC analysis predicts a sharp downturn in cases by July. But with vaccination rates stagnating, there’s work to be done. More than 57% of U.S. adults have started a COVID-19 vaccine course, and more than 110 million are fully covered, according to CDC. President Joe Biden now wants to reach a goal of 70% adults starting a course, and 160 million Americans completing immunization, by Independence Day.
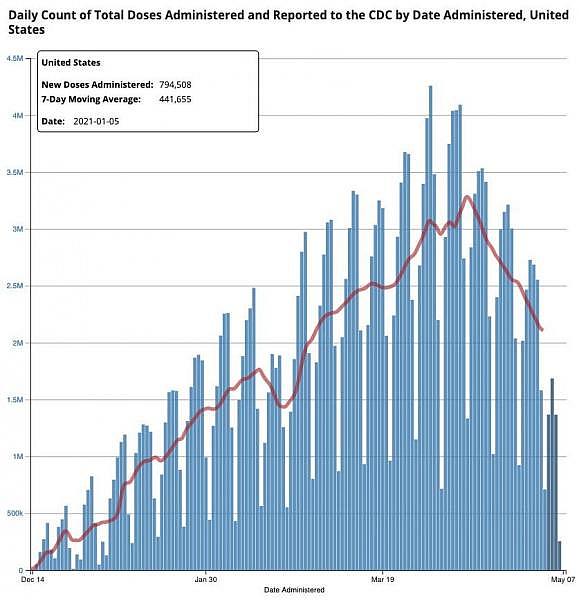
That could be a challenge; the latest poll from the Kaiser Family Foundation indicates that just 9% of the population is still eager for a vaccine but hasn’t had one yet. “There are millions of Americans who just need a little bit of encouragement to get that shot,” Biden said. So vaccine programs are making changes. For the federal distribution program, that means if a state doesn’t order its full weekly share, those doses will be made available to other states that want them. The administration is now sending vaccines to rural clinics, and investing about $730 million in outreach efforts, reports STAT. The “long grind” to community vaccination means knocking on doors, mobile clinics, and vaccines provided by family doctors, writes Ken Alltucker at USA Today. The new approach should improve vaccine access in underserved communities, reports The New York Times. Dr. Karen Landers, state health officer in Alabama, told the Times, “We are not giving up.”
Pfizer vaccines for kids coming
- “We still don’t know who the coronavirus’s victims were,” by Ibram X. Kendi, The Atlantic
- “Inmates sent home during COVID-19 got jobs, started school. Now, they face possible return to prison,” by Kristine Phillips, USA TODAY
- “Reaching ‘herd immunity’ is unlikely in the U.S., experts now believe,” by Apoorva Mandavilli, The New York Times
- “The logic of Biden’s new July 4 vaccination goal,” by German Lopez, Vox
- “Why a former anti-vax influencer got her COVID-19 shot,” by Tara Haelle, Texas Monthly
Events and Resources
- May 11, 4 p.m. PT: Science Writers in New York interviews NIH scientist Dr. Robert Seder about COVID-19 vaccine strategies, development, and efficacy.
- May 13, 9:30 a.m. PT: The second in a series of webinars on COVID-19 vaccines will focus on immunization for children and teenagers.
- May 13, 10 a.m. PT: The Wilson Center hosts a webcast on how COVID-19, racism and disinformation have contributed to the Asian American experience during the pandemic.
- May 21, 11 a.m. PT: With the pandemic highlighting ongoing disparities in health care for people of color, the National Institute for Health Care Management Foundation will present “Achieving health equity: What’s next?” (The Foundation also supports Center for Health Journalism webinars.)
- SciLine offers a number of resources for reporters, including expert quotes; recent topics include post-vaccination risks and masking.

