Q&A: How a Detroit News investigation uncovered years of dirty surgical instruments and at-risk patients
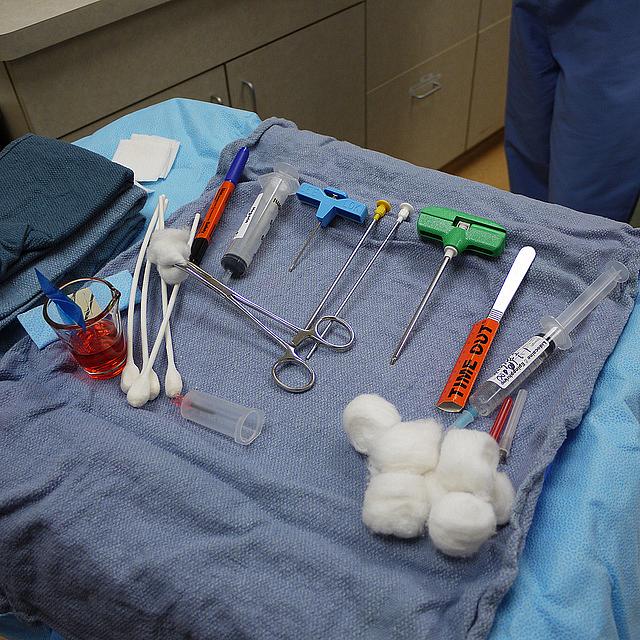
Photo: Thirteen of Clubs/ Flickr
When an anonymous source informed The Detroit News that the Detroit Medical Center (DMC) was exposing patients to improperly sterilized surgical equipment, health care reporter Karen Bouffard and an investigative team set out to uncover the full scope of what turned out to be a long-running problem. As a result, the state of Michigan has launched a probe into how the center sterilizes instruments, an issue doctors there have complained about for years. We recently caught up with Bouffard, a 2013 National Fellow, to ask her how her team conducted the investigation, and what tips she has for reporters covering similar ground.
Q: How did your team obtain the emailed complaints and other records that informed your reporting?
A: I was given a little more than 200 pages of documentation from an anonymous source that reached out to me.
Q: How did you substantiate the information?
A: The information we received was absent of personal information on specific patients. We had three small leads that we were able to track down on one 7-month-old child exposed to bio-burden during open-heart surgery. It took months. Members of the population this health system serves are often very poor, have multiple phone numbers and addresses, and many live with relatives. We first found the father, and then I tracked down the mother through relatives who lived in Tennessee (found through Lexus Nexus). A neighbor of one of the mother’s relatives gave us the phone number of the grandmother of the baby. The mom was only willing to communicate through Twitter. She was the custodial parent, so it was critical to get her permission to name the child and talk specifically about her case. The other cases in the documents just provided general information.
We had copies of internal reports and access to numerous inter-office emails between people who worked with DMC, describing problems that had gone back 11 years. A lot pertained to specific incidents where patients had been exposed to bio burden from instruments that had been labelled as sterilized.. Although only a fraction of the occurrences from our original documents were reported officially, we saw that many of the incidents referenced in the emails were actually reported. So we were able to crosscheck between bodies of information and confirm that they were the same.
Another thing we were doing the whole time is filing FOIAs on all the inspections, reports, and complaints in all of the hospitals in the city of Detroit. We did this to compare and know that this is a problem as opposed to a general issue that exists within the entire industry. DMC by comparison had deeper, broader problems, worse than would be expected at any other hospital.
Q: At what point did you share your findings with the health system? How did they respond?
A: Two months before publication we contacted the health system and described some of the instances from our information, letting them know the scale of what we had. They scheduled an interview with us immediately. The very next day they announced that they had signed a contract with an outside group to take over management of sterile processing. They sent the CEO to talk with us a few days later, and agreed to speak with us on record. They didn’t deny problems, but didn’t address specific incidents. The chief administrative officer did say it had been an issue they’d been working on for many years, and that they were hiring an outside management company to fix it. That was a pretty big confirmation that what we had was accurate.
Q: Can you describe your reporting process for this series?
A: The reporting throughout was very challenging — no one would speak with me at first. There was a lot of information contained in the documents, so Joel Kurth, editor of our investigations team, eventually joined me. We ended up making Excel sheets with pertinent information on them as we collaborated. We had 99 people to reach out to that were mentioned in the emails, and we were trying to contact past employees who were willing to talk, former executives who had left, former union employees, people who had been fired, and anyone who was no longer associated with the health system. No one who was currently working there would speak with us on the record.
We finally got four surgeons who would speak anonymously, who confirmed everything in the emails. If we hadn’t had a number of anonymous sources, we wouldn’t have gotten very far. We had to make sure everything we had was legitimate.
We also spoke with experts in sterile processing and people who could talk to us about pathogens and the degree of danger for patients being exposed to bio burden who could go through the sterilization process and survive. We learned that even if there aren’t live pathogens, the body can have a very severe reaction to foreign matter that is introduced deep inside the body in the surgery process. The autoimmune response can lead to sepsis and full organ failure in the body.
So we had to learn the whole landscape: the science, the legalities, federal and state regulations, and the regulatory agencies and organizations that research patient safety issues and make policy recommendations.
Q: Do you have any tips for journalists who are encountering difficulty in getting sources to speak on the record?
A: I think that in the absence of having more people willing to go on record, we really had to find out if the information we did have was factual, and if we could use it.
Don’t give up: it took us a really long time to reach out to sources, sometimes several times through email. Some of the people who ended up speaking with us completely ignored us on email. In many cases, reaching out on Twitter and Facebook was the only way. You have to really keep working at it; some of these people did end up talking, but only after the 4th or 5th attempt.
Because those involved with the sterile processing at DMC refused to speak with us at first, we talked to people who are experts or work in the field, who gave us vital information on standards and how things are supposed to be done.
Q: Do you plan to pursue this story further? Are there any angles you still wish to explore?
A: [There’s now a] 90-day waiting period to see if DMC can fix its issues. If not, their Medicaid billing could be revoked, but that’s such a rare occurrence that it probably won’t happen. Nursing homes can be fined by CMS under federal regulations if they are found to be not in compliance, and can suffer significant financial damage if they don’t follow rules and standards. In the case of hospitals, there is no deterrent. CMS’s only recourse is to withdraw future Medicaid funding. In DMC’s situation, since it’s a safety net system that is very much needed in its community, it would be extremely drastic for Medicaid to withdraw funding or keep it from operating.
So without a deterrent such as the ability to fine for past transgressions, what keeps hospitals at task in a situation where you have a long history of trying to fix the problem but not succeeding? And is this 3-month period long enough to ensure they do everything that’s necessary to fix the problems and ensure patient safety?
Q: Many have called for more transparency in health care and better notification of potential risks to patients. What role can journalists play here?
A: One way journalists can help is by reporting on the health systems that are doing things the right way. I think there are some examples of health systems that are much more transparent. For instance, I reported in my series that Seattle Children’s Hospital had a similar problem, discovering that there had been an issue with instruments going through the sterilization process and coming out infected and being used on children. The difference was that Seattle Children’s Hospital called a press conference and offered free blood testing for the children. Showing how hospitals can do it right is one way journalists can demonstrate to the hospitals that they’re working with and the communities they live in that there are better ways that things can be done.

